THE ELECTRON - MORE MYSTERIOUS THAN YOU IMAGINED
By Michael Harwood
Electrons are so common that people take them for granted. In this essay, I am going to look at electrons in more detail and examine their brilliant design. At first glance, electrons are not that special, just tiny negative particles that make up atoms
PART I: PROPERTIES OF ELECTRONS
There are about 100 elements that everything in the known universe is made of. Each element is made up of atoms of that element (e.g. gold is made of gold atoms). One would think that there are 100 basic particles - one for each atom. Strangely enough, atoms, in turn, are made up of three more fundamental particles: protons and neutrons in the nucleus, and electrons orbiting the nucleus in some sort of cloud-like distribution. There is no difference between the protons, neutrons and electrons of one type of atom and that of another type. What then makes the atoms of each element unique and identifiable with distinct chemical properties? The number of protons determines the number of electrons in a neutral atom. Neutrons are needed to hold the protons together in the nucleus. However, neither protons nor neutrons take part in any chemical reactions or form chemical bonds - it is only the electrons that do.
Charge
The electron has exactly the same charge as the proton, but with the opposite sign. There is no obvious explanation how this came about, since electrons and protons are completely different types of particles. I am not sure exactly how important the equality of charge is in cosmology and subatomic physics, although the numerical value of the electron charge is critical in quantum electrodynamics (see below). At first glance, one can simply say that if the electron had slightly more charge than a proton, then objects would be negatively charged and repel each other.
There is undoubtedly some much more fundamental reason why it is crucial electrons and protons have exactly the same, but opposite charge. The difference between the charge of an electron and a proton is less than 1 x 10-21 ! i ii
Mass
The proton has 1836 times the mass of an electron. Why is this so? No one knows. Because electrons have a mass, they have particle-like properties and so are subject to Newton's Laws of Motion (F = ma). They can change speed and accelerate - which is important for producing radiation. Only massless particles like photons go at a constant speed.
It is important that the electron be lighter than the nucleons, so that it can orbit the nucleus. If electrons were 2000 times heavier than protons, there is no way that they could orbit nuclei. The masses of the proton, neutron and electron are very carefully arranged in just the right way. The mass of the neutron is slightly more than the combined masses of the proton and electron. This allows a neutron to decay into a proton, an electron and a neutrino. If this were not the case, and the mass of the neutron was exactly equal to the combined masses of the proton and electron, then all the protons and electrons of the primeval universe would simply have combined into stable neutrons. The result of this is that there would not have been enough hydrogen lying around to act as the fuel for stars.iii If the neutron weighed a lot more than a proton + electron, then neutrons would decay more readily and there wouldn't be enough neutrons to make larger stable atoms - perhaps just hydrogen and helium.
Electrons are affected by three of the four fundamental forces that define the nature and interaction of everything in the universe: gravitation (due to its mass), the electromagnetic force (due to its charge), and the weak nuclear force (involved in neutron decay).
Astronomers have shown that the proton-electron mass ratio has been constant for at least 6 billion years all over the universe. iv
Size
As far as we know, while an electron has mass and charge, it has no size. A electron is an infinitely small particle - and yet, due to its charge and movement around a nucleus, electrons are responsible for most of the volume of atoms!
Type of particle: Leptons
Electrons are a type of particle called leptons. The other type of particles with mass is called hadrons. Hadrons are made up of quarks, but leptons are not made up of anything smaller. They have no internal structure. Each quark has the charge of exactly 1/3 of an electron charge - giving the proton the equal and opposite charge of an electron – even though hadrons and leptons are utterly different from each other. This exact correspondence of charges has never been explained.
There are three generations of leptons just as there are three generations of quarks (but again, no one knows why exactly three). The lepton families or flavours are electrons, muons, and tau particles. Each one of these has its own type of neutrino. The masses of these particles are: electron = 0.511 MeV ; muon = 105.7 MeV, tau = 1777 MeV v
These masses follow a very simple formula called the Koide formulavi . This formula has never been explained nor understood.Lepton Number
All leptons have a number called a lepton number. Quite simply, all leptons have a number of +1 and their antiparticles have a lepton number of -1. The conservation of lepton number is just needed to explain why an antineutrino is the type of particle that must be produced when a neutron decays and produces an electron (there has to be some 3rd particle to handle conservation of momentum). Lepton numbers are conserved within each of the 3 families - except for neutrino oscillations. vii
Spin
Electrons, like all fermions, have a spin of ½ . The most important result of this is the Pauli Exclusion principle which states that no two fermions can exist in identical energy quantum states (i.e. with the same quantum numbers). This is critical for how electrons fill orbitals in atoms, creating different elements.
Having a non-integer spin allows two types of handedness (chirality): left- and right- where the spin is opposite or aligned with the momentum of the electron. Strangely enough, only left handed particles can interact with the weak force - which means that all neutrinos are left handed. (Electrons can change their handedness, but it is not clear how neutrinos would be able to do this.)
Magnetic moment
Electrons act like tiny magnets. This property is thought to be due to their spin - except that spin does not mean that they are really spinning. So this picture of the origin of their magnetic field doesn't actually make sense. Electrons have a magnetic moment that is an astonishing 658 times that of the proton which is much larger and heavier than an electron!viii This large value is important for some types of magnetism (see below).
Origin
The origin of electrons, like all other fundamental particles is unknown. It is assumed that they were created somehow in the Big Bang. Electrons can also be created via neutron decay:
10n → 11p + 0-1e + 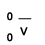 Neutrons → proton + an electron + an antineutrino.
Neutrons → proton + an electron + an antineutrino.
A second method by which electrons can be created is by gamma rays interacting with a nucleus. The high energy gamma ray makes an electron along with its antiparticle, a positron. Each positron will annihilate itself with another electron producing two more (weaker) gamma rays, so there is no net gain in electrons from this method. (One of the unsolved mysteries of the universe is why there is so much more matter than antimatter.)
Antielectrons and QED
Like all fermions, electrons have an antiparticle. The antiparticle of the electron is called the positron (also known as the anti-electron). Possibly the most accurate theory in physics is quantum electrodynamics (QED). It describes how electrons, positrons, and photons interact. And yet, "the theory of QED still produces mathematical infinities in certain contexts. ... These infinities suggest that QED is not by any means a final theory-ix In QED, the electron mass, electron charge, and electron magnetic moment are all used to determine the nature and strength of the interaction between electrons and photons. If any of these numbers were even slightly different, the whole universe would be completely different.
Electrons as waves
De Broglie applied Comptons's theory of wave-particles to electrons. He showed that electrons can have a wavelength. (This property is used in electron microscopes.) The wave nature of electrons is also important in atoms. Electrons are often thought of as particles, but at a more fundamental level, electrons orbiting a nucleus are represented as wavefunctions. They have wavelike properties and these wavelike electrons interfere with each other.
When an electron orbits a nucleus, it sets up a standing wave pattern. There are only certain standing wave frequencies and energies that are allowed. Electrons are forbidden from areas where they do not make a complete standing wave around the atom - otherwise they would interfere with themselves and cancel themselves. This is the basis of quantum theory and explains why electrons do not radiate energy as they orbit the atom. The energies are quantized.
The possible locations of the electron are described by four quantum numbers. The interplay of these numbers determines the properties of each element. (more below in "Electrons in Atoms")
Is there any proof that the electron actually orbits the nucleus or is it rather just a standing wave? The only experimental evidence that indicates that an electron might actually orbit a nucleus (as a planet orbits the sun) is the London force.
Electrons as probability clouds
Because electrons are so small and have a very small mass they are subject to quantum mechanics and in particular, the Heisenberg uncertainty principle. This says that the exact momentum and position of the actual electron cannot be simultaneously determined. In other words, the more accurately one knows a particle's position, the less accurately one can know its momentum, and vice versa.x This now leads to the Schrodinger model of the atom where electrons are described as probability clouds of various shape. These cloud like probabilities are 3D regions of space where an electron is most probably located and are known as orbitals. Each orbital can contain two electrons which must have opposite spin. The whole volume of the atom is made up of the orbitals of the electrons.
Electron Orbitals
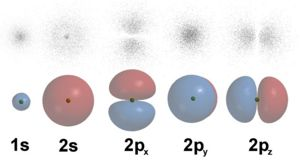
Notice that there are some gaps in the probability clouds. An electron in the 2pz orbital may be in the blue region or in the red region, but never exactly between them on the y-axis. How does an electron cross from the left side to the right side without ever being in the middle? A particle can't do this, but an electron as a standing wave can. (If electrons were large enough to not be subject to quantum mechanics, chemistry would be completely different.)
Electrons in Atoms
For some reason, there are certain electron configurations that are stable. Very early on, it was realized that electrons are located at different energy levels. The inner energy levels hold fewer electrons and must fill up first. The first energy level can only hold 2 electrons, the next 8. When the outermost energy level is full, the atom is stable. The drive for stability, for full energy levels, is what causes chemical reactions to occur.
As mentioned earlier, there are 4 quantum numbers that determine the location of electrons. The first of these, the principal quantum number n, determines the main energy level. The second of these, the angular quantum number l, determines which orbital the electron is in. These are commonly referred to using the letters s, p, d, f, g, instead of using numbers. The other two quantum numbers, describe how many sublevels are in each orbital, essentially saying that an s orbital can hold 2 electrons, p = 8, d = 10, and f = 14 electrons.
Generally only electrons in the outermost energy level take part in bonding and these tend to be the s and p orbitals. This means that normally there are a maximum of 8 valence electrons. Thus there should be only 8 types of atoms (for example, all of the halogens should act the same way; and C, Si, Sn and Pb should be identical except for density). If one adds in the 10 'd' electrons and the 14 'f' electrons which may be part of the valence level, then there are a total of 32 different numbers of valence electrons (corresponding to the 32 columns of the periodic table). In every atom the electrons fill up the 1s, then 2s, 2p, 3s, etc orbitals in the same sequence. Why are there more than 32 different atoms? It is because the locations or values of the energy levels are different and so are the sizes of the atoms. The 1s level has different energy in lithium and in cesium. Li and Cs also each have 1 valence electron, however, Li holds onto it much more tightly as lithium is a much smaller atom.
The Pauli exclusion principle states that no two electrons can have exactly the same set of 4 quantum numbers. The Pauli exclusion principle is so strong, that it prevents white dwarfs from collapsing further. xi If the gravitational pressure is extremely strong, gravity will squeeze protons + electrons together to make neutrons. This creates a neutron star.
It is a peculiar feature of quantum physics that these quantum numbers which restrict the energy and location of an electron, only appear when an electron is bound to a nucleus, forming an atom. A free electron has none of these limitations that are essential to creating atoms.
PART II: WHAT ELECTRONS DO
Elements
The different numbers of electrons in atoms and the different energy levels for the electrons around the atomic nucleus are the only things that produce the fundamental distinctions between helium, lead, titanium, oxygen, and other elements. The number of protons is important only in that it determines the number of electrons and changes the spacing of the energy levels that the electrons are in. Adding electrons is not like adding neutrons - just making isotopes with virtually no change in properties. Electrons determine the properties of each element. As soon as there is more than one electron, things get extremely complex with the interaction between the individual electrons and between each electron and the nucleus, remembering that the electrons will be in slightly different energy levels. When one adds in interactions with electrons in neighbouring atoms the complexity increases dramatically and it is very hard to predict the properties of the elements by their number of electrons (e.g. why are gold and copper coloured, but other metals are not).
Chemical Bonds
All the different types of bonds between atoms are due to electrons. If electrons did not form bonds, no atoms would ever be joined together. That means that there would be no molecules, no liquids, and no solids, only monatomic gases.
There are 5 different types of bonding between atoms. Almost all of these bonds can have varying strength depending on the elements involved and the bond lengths.
Covalent bonds are directional and form between non-metals. They allow complex biological molecules like DNA and proteins. Covalent bonds can be incredibly strong as in diamond, or quite weak as between graphite layers. Covalent bonds are extremely important in all organic molecules. Most covalent bonds allow rotation about the bond. The peptide bond between C and N in amino acid chains is a bond that does not allow rotation. This is vital for life because each chain must form into a specifically shaped protein molecule and not flop around uselessly. Covalent bonds are typically no broken by water (so covalent molecules are not destroyed by water).
Alternating single and double covalent bonds in carbon chains are responsible for the colours produced by synthetic dyes, by organic molecules like chlorophyll, hemoglobin, and by the colours of vegetables, flowers, and leaves in the fall.xii
Ionic bonds are also very strong, but are non-directional. Ionic bonds form between a metal and a non-metal (or polyatomic ion). They build 3D structures and are responsible for most rock-forming minerals. Ionic substances have very high melting points (e.g. rocks, salt). Many ionic bonds can be broken by water, meaning that they can dissolve in water, but many ionic substances are insoluble.
Metallic bonds also have very specific and unusual characteristics and are responsible for all of the unique properties of metals.
"When metal atoms combine with each other, the outermost electrons lose contact with their parent atoms. The remaining positively charged atomic centers form an ordered structure while the outer electrons move freely around the whole sample. These freely moving electrons, called conduction electrons, can carry heat energy and electric charge easily throughout the metal, making metals good conductors of heat and electricity." xiii
Van der Waals bonds are the weakest and can often be disrupted by thermal energy. These are bonds between electrically neutral molecules or atoms. There are two subtypes: dipole-dipole (e.g. between polar HCl molecules), and London forces (from transitory dipoles e.g. in Xe or solid H2). Van der Waals bonds are important for viscosity, surface tension and when two surfaces come in contact. Many of the properties of plastics and polymers are dependent on these bonds.
Hydrogen bonds are formed when a hydrogen atom is sandwiched between two atoms which attract electrons (like O and N). Hydrogen bonds are actually a type of dipole-dipole bond, but are distinct enough to be given their own name. They are extremely important for the exceptional properties of water. Hydrogen bonds are used to hold the two sides of DNA together. They are weak enough that the two strands can be unzipped and zipped up without damaging the rest of the molecule.
All the physical properties of solids - hardness, transparency, phase, colour, thermal conductivity, heat capacity, etc. - are due to the types of chemical bonding which in turn depend on the properties of the electrons orbiting a nucleus. The different elements and different chemical bonds - both caused by the properties of electron clouds around nuclei - are the reason matter comes in such incredibly diverse forms to make mountains, water, living organisms, and chewing gum.The theory of quantum electrodynamics is needed to explain bonding in more detail because it gets so complex (in particular the attractive forces between electrons and atoms, how they add up and vary with distance, hybridization of orbitals, etc).
Chemical reactions
All chemical reactions are based on electron bonds breaking and reforming. If electrons did not form bonds that could break, there would be no chemical reactions. (These reactions can also be thought of as electrons being transferred, requiring or releasing energy). Chemical reactions include digestion, photosynthesis, combustion, respiration, as well as all the chemical reactions in industry (glues, paints, cement, batteries, etc.). Some biochemical reactions, in particular photosynthesis, rely on the transfer of individual electrons from one molecule to another. The electron is energized by a photon of light, and as it releases its energy the chloroplast uses the energy to make ATP molecules.
Electricity
Electricity is simply the movement of electrons through a conductor. Normally electricity is used as a way of transferring energy from one location & form to another (e.g. chemical energy from a battery to radiant energy in alight bulb). The various ways of producing electricity typically involve giving energy to electrons (batteries) or applying a force to them (generators). Electricity is used for so many purposes that there is no point trying to list them here.
Magnetism
The most powerful form of magnetism is the everyday magnetism known as ferromagnetism. This is caused by unpaired electrons having their spins aligned over large regions of the material, which requires quantum physics to explain properly. The orbital motion of electrons causes diamagnetism and paramagnetism which are much weaker than ferromagnetism. Moving charges create a magnetic field — which is the principle used to make electromagnets.
Eletromagnetism is crucial for the operation of generators, motors, transformers, loudspeakers, hard drives, doorbells, and compasses.
Light
Electrons are responsible for light. When any charged particle accelerates, it emits electromagnetic radiation - radiating away energy. This is how radio waves, microwaves, light, etc. are produced. Strangely enough, electrons never radiate energy when orbiting a nucleus, even though circular motion necessitates continuous centripetal acceleration.
- Electrons produce visible and UV light when they jump from one energy level to another in an atom. The difference in energies is just the right size to produce visible light. Larger jumps produce UV.
- Every element has its own unique line spectrum of light that is emitted.
- To get X-rays, an inner electron is knocked out of the inner most shell of a large metal atom (e.g. copper). One of the valence electrons drops down to fill the hole, emitting an X-ray as it does so.
- Some of the smaller jumps between energy levels produce IR, but IR can also be produced by whole molecules moving or rotating.
- Electrons produce microwaves as they are bunched up and forced to oscillate in a magnetron.
- Electrons produce radio waves when they are forced to oscillate in an antenna.
- Electrons allow mirrors to exist and cause the silvery reflective surfaces of metals. When light hits a metal the electron oscillates at the exact frequency of the light. As it does this, it absorbs the incoming photon and emits another identical one.
Other applications
- Lasers are made by controlling precisely how electrons jump from one energy level to another.
- Electron beams are used in CRTs (i.e. TV and computer monitors), electron microscopes, photomultiplier tubes, and synchrotron radiation.
- Plasma displays and fluorescent lights are due to electron excitation.
- Electric fields are produced by moving electrons via high voltages to produce unbalanced charged surfaces. These fields are used to control LCD screens, particle accelerators, electrostatic precipitators, and many other applications.
- Minute electric fields inside semiconductors are used to create transistors, solar cells, and LEDs.
SUMMARY
The importance of electrons to all areas of chemistry and physics is incalculable. The applications of electrons in technology are limitless and wide-ranging beyond belief.
Consider this: Assume that you had the ability to create fundamental particles. How would you go about creating a particle that does all of the things that an electron does - and yet it has no size! One has to consider the electron as a wave, a particle, and a probability distribution. I would have enough trouble merely creating a mechanical device, for example, a regulator for compressed gas tanks, and that only has to do 3 very similar functions. The complexity and properties of an electron are astounding and mind-boggling. I simply cannot believe that the properties arose by chance because of the suitable type of universe that happened to somehow bring itself into being. It seems much more likely that there was some incredible intelligence behind the design of an electron. Just like the fine-tuned universe, electrons point to a creator that exists outside the universe.
Endnotes Click on the Endnote number to return to your place in the text
i http://hepwww.ph.qmul.ac.uk/~pipk/PIPK-Unpublished-jottings/Are%20Atoms%20Neutral.doc
ii "An upper limit to the charge difference between the proton and electron, defined by f=1+(electron charge )/(proton charge ), was found to be ƒ≤0.8×10-19. It was necessary to assume: (neutron charge)= (electron charge) + (proton charge)." http://prola.aps.org/abstract/PR/v164/i5/p1599_1
iii http://www.geocities.com/paulntobin/finetuned.html
iv "Although most other molecules rotate faster when they absorb the energy from radio waves, ammonia actually flips inside out, with the nitrogen moving from above the hydrogens to below. This flipping depends strongly on the ratio between the mass of the proton and the electron. Knowing this, Henkel's team compared their ammonia data to other molecules in the same galaxy and found the ammonia absorption had not significantly shifted from where it was expected to be."http://www.space.com/scienceastronomy/080714-mm-proton-electron.html
v 1 MeV = 1.78 x 10-30 kg
vi http://en.wikipedia.org/wiki/Koide_formula
vii Neutrino oscillations mean that for some reason, neutrinos can switch back and forth from one type of neutrino to another. Electrons are unable to do this. The physics behind these oscillations is still being worked out.
viii This number comes from dividing the magnetic dipole moments as listed http://en.wikipedia.org/wiki/Magnetic_moment Comparing the magnetic moment of protons and electrons gives about a factor of 900 (using values from Wikipedia and changing nuclear magnetons to Bohr magnetons).
ix http://www.wisegeek.com/what-is-quantum-electrodynamics.htm
x http://en.wikipedia.org/wiki/Electron
xi http://hyperphysics.phy-astr.gsu.edu/hbase/pauli.html#c2
xii Structure and Colour in Dyes: http://stainsfile.info/StainsFile/dyes/dyecolor.htm
xiii "Electron," Microsoft® Encarta® Online Encyclopedia 2008 http://encarta.msn.com
April 2013: converted from WORD to HTML. Minor edits.